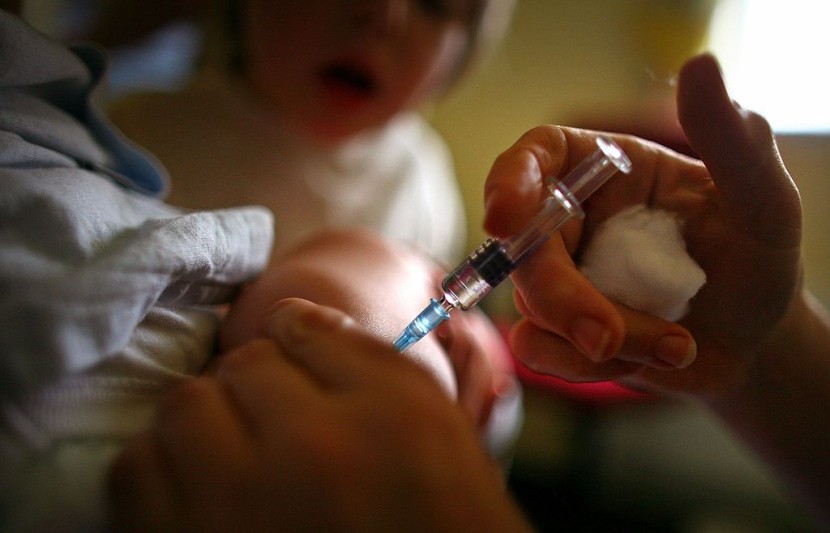
Sixteen infants recently underwent Phase 1 of the clinical trial of Pfizer's COVID-19 vaccine, and an 8-month-old boy from New York is the youngest among the recipients.
Pfizer's COVID-19 Vaccine Clinical Trial
Nearly 140 million American adults have received at least one injection of a COVID-19 vaccine, bringing the nation a step closer to immunity. Vaccine manufacturers Pfizer and Moderna have gone on to the next phase of the virus war, testing to see whether the vaccine is safe and successful for children.
According to a recently published article in the Daily Advent, Pfizer-BioNTech began its clinical trial on infants to determine the efficacy and the proper dosage of the vaccine within the age group.
Dr. Yvonne Maldonado, professor of pediatrics, epidemiology, and population health at Stanford University, said that letting children receive the COVID-19 vaccine will help reduce the number of infections, according to an article in ABC News.
The Youngest Recipient of COVID-19 Vaccine
An 8-month-old boy from the upstate Baldwinsville, NY, is the world's youngest recipient of two doses of the Pfizer COVID-19 vaccine among the 16 infants, according to a recently published article in The New York Post.
Upstate Medical University gave baby Vincenzo "Enzo" Mincolla his second shot on Wednesday as part of a clinical trial. The hospital is one of four sites in the United States where the Pfizer vaccine is being tested in children under the age of five. Currently, the U.S. is the only nation running such experiments.
The baby received his first shot three weeks ago when he turned eight months old. Dr. Joseph Domashowske, an upstate pediatric infectious disease specialist leading the trial, told a news outlet that he is the trial's youngest recipient. Five other babies are taking part in the upstate experiment.
The Parents Release a Statement
The infant's parents are doctors. They both said, "We both feel it's important to end this pandemic, and the quickest and safest way is to vaccinate our way out of it."
They also shared that their baby did not manifest any qualms after receiving the vaccine. In fact, their baby slept and ate well. When asked if the baby experienced side effects, they said their baby did not experience any fever or chills.
The doctors also shared that they told other parents who are hesitant to let their children receive the vaccine that they allowed their son to receive the first dose. This is to boost the confidence of other parents as well.
Meanwhile, Pfizer-BioNTech temporarily halted its trial after Phase 1 to determine the vaccine's efficacy to the identified age group. Additionally, the move is also aimed to determine the right amount of dosage for infants.
As of this time, Pfizer said that infants who receive the vaccine under the clinical trial are all well, and there were no reports about these infants experiencing side effects. Results of the clinical trial are significant for other vaccine developers because not all COVID-19 vaccines suit a particular age group.
Related Article : Mixing COVID-19 Vaccines Is Link to More Side Effects, New Study Shows
© 2025 HNGN, All rights reserved. Do not reproduce without permission.








