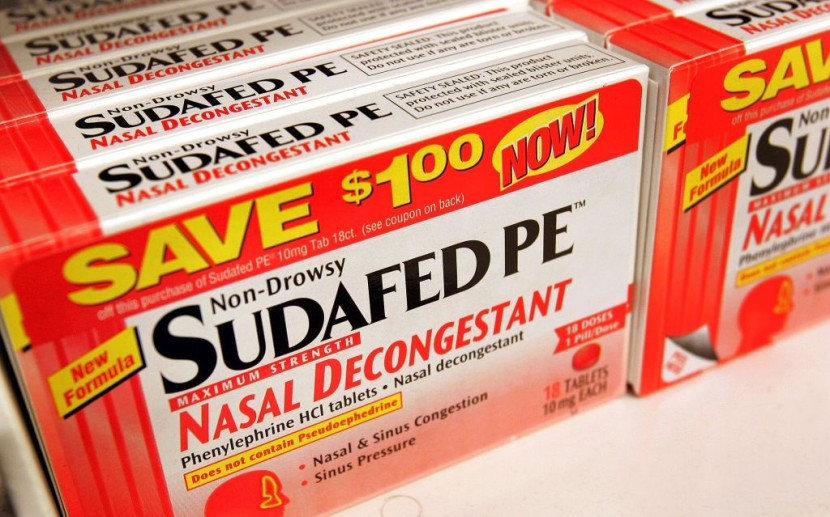
Millions of Americans turn to the most popular decongestant whenever they need to clear their stuffy nose. However, government specialists who evaluated the most recent study on the controversial medication component say it is ineffective and is probably no better than a placebo.
Improved Evaluation of Phenylephrine
On Tuesday, February 12, the Food and Drug Administration's (FDA) advisory committee voted unanimously against the efficacy of the active component in several over-the-counter cold and allergy treatments, such as Sudafed, Allegra, and Dayquil.
According to Dr. Mark Dykewicz, an allergy expert at Saint Louis University School of Medicine, phenylephrine used in the mentioned medications has not been shown to alleviate congestion in recent, well-conducted trials.
AP News reported that since the older component called pseudoephedrine was taken out of over-the-counter decongestants, the FDA convened its outside experts to take another look at phenylephrine, the major medication in OTC decongestants. The shift was necessary due to legislation passed in 2006 since pseudoephedrine may be converted into methamphetamine via illegal methods.
Even while nonprescription versions of Sudafed and similar medications still exist, they only make up approximately a fifth of the $2.2 billion market for oral decongestants. The remainder are phenylephrine variants, denoted by the label "PE" on some packaging.
Potential Product Recall?
If the FDA adopts the panel's recommendations, manufacturers, including Johnson & Johnson, Bayer, and others, may be forced to recall phenylephrine-containing oral drugs from stores. According to CBS News, that would compel people to buy the tablets online or from pharmacies or to use phenylephrine-containing nasal sprays and drops, none of which are currently being evaluated.
Researchers from the University of Florida called for this week's two-day conference by petitioning the FDA to remove phenylephrine products after finding that they were no more effective than placebo tablets in relieving congestion in those suffering from colds and allergies.
Similar concerns were raised about the drug's efficacy in 2007, but the FDA nonetheless permitted sales to continue while it reviewed the data.
This time, all 16 members of the FDA panel agreed that the available data does not support the drug's effectiveness.
Jennifer Schwartzott, who served as the panel's patient representative, felt that the drug's oral dosage should have been withdrawn from circulation long ago. "Patients require and deserve medications that treat their symptoms safely and effectively and I don't believe that this medication does that."
The advisory panel mostly agreed with the findings of an FDA scientific study issued in advance of this week's meeting, which identified multiple faults in the studies used to justify phenylephrine's approval in the 1960s and 1970s. In their words, the studies were "extremely small" and made use of outdated statistical and research methods that are no longer tolerated by the government.
© 2026 HNGN, All rights reserved. Do not reproduce without permission.








