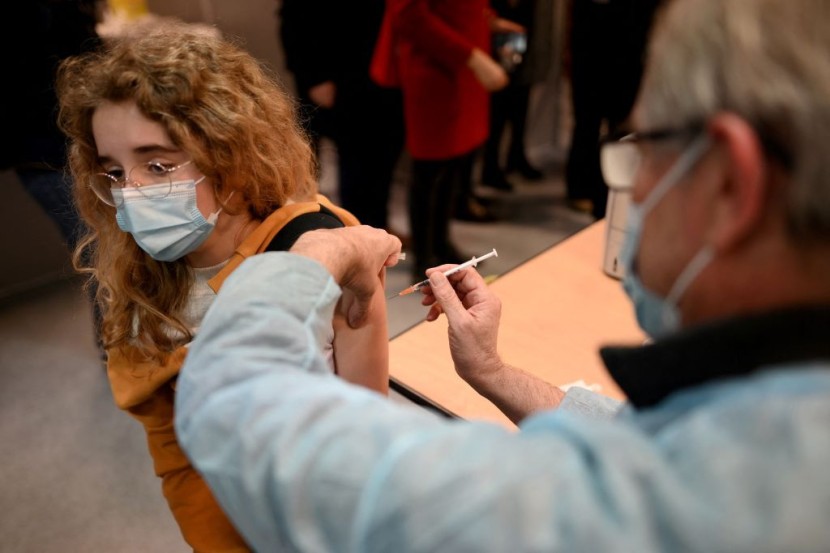
The Food and Drug Administration (FDA) announced new measures for 12 to 15-year-olds who are already fully vaccinated.
According to reports, the FDA authorized children within this age group to also receive their booster shots or a third dose of Pfizer-BioNTech's coronavirus vaccine.
Initially, those who wish to get their booster shots need to wait six months before doing so. But the FDA also shortened the waiting period for children from six to five months.
FDA still waiting for CDC's approval
However, it is important to note that the Centers for Disease Control and Prevention have not yet approved the FDA's authorization.
On Dec. 9, the FDA authorized booster shots for 16 to 17-year-olds, and the CDC approved the authorization on the same day. However, the same has not happened for the FDA's authorization for 12 to 15-year-olds.
The FDA also announced this week that children aged 5 to 11 could also get their booster shots if they are immunocompromised. This is because two doses may not be enough to protect an immunocompromised child from COVID-19.
FDA director explains the importance of booster shots
Dr. Peter Marks, the director of the FDA's Center for Biologics Evaluation and Research, said that they made the recent authorizations amid the belief that a booster shot can help provide better protection against delta and omicron variants.
"In particular, the omicron variant appears to be slightly more resistant to the antibody levels produced in response to the primary series doses from the current vaccines," he said via the Huffington Post.
Marks and acting Commissioner Janet Woodcock also said that the data on myocarditis, which has been linked to the mRNA vaccines among young men, is rarer with a third vaccine shot. It is also particularly rare among people within the 12 to 15 age group.
According to ABC News, the FDA is also working towards ensuring that they can also administer the booster shots from other vaccine brands safely among children.
The goal is to get the J&J booster shot two months after the first shot, the Moderna vaccine six months after the second shot, and the Pfizer booster five months after the second shot.
Active COVID-19 cases in the US reached 1 million this week
According to USA Today, there is a growing concern regarding the increasing number of COVID-19 cases in the country. Following the recent New Year's weekend, the numbers already reached over 1 million on Monday.
A data from Johns Hopkins University also revealed that one in every 100 Americans had been reported as a positive case in just the last week.
Joe Biden won't announce a countrywide lockdown
Joe Biden and Kamala Harris have plans to meet with the White House's COVID-19 response team to discuss their next steps to help prevent the further spread of the virus.
Some states announce mask mandates, especially in schools. There are also other restrictions in place, but no one has announced a hard lockdown as press writing.
Last month, Biden said that he doesn't have plans to announce a countrywide lockdown, and he won't also expand vaccine mandates despite the alarming number of COVID-19 cases in the country, according to CNBC.
© 2026 HNGN, All rights reserved. Do not reproduce without permission.








