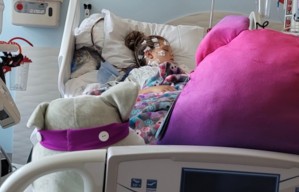
A drug that was unapproved by the Food and Drug Administration saved the life of a 4-month-old girl in Seattle.
Tatiana, the infant, inhaled meconium - which is amniotic fluid mixed with her stool - while she was in her mother's womb, causing her lungs to collapse. The medical emergency was caught by doctors at her birth who warned Tatiana's parents they may lose their daughter, reported CBS News.
"I wasn't going to give up on my daughter," Tatiana's dad, Bruce Saiaana, told Seattle's CBS affiliate KIRO.
Tatiana was immediately put on an ECMO (extracorporeal membrane oxygenation) machine, which kept her heart and lungs going for about a month. The machine was keeping her alive, but doctors were unsure if the infant would ever be able to survive without it because her lungs collapsed before they were fully developed, reported KIRO.
The only resource left to try was a drug called perflubron, which is an oxygen-rich liquid that fills and expands the lungs before evaporating, reported CBS News. The only issue was that the drug was never approved by the FDA and it's manufacturers found it did not provide enough benefit as a liquid ventilator in the 1990s.
Tatiana's doctors received a waiver to use the drug, which gave them one last hope to save the patient's life. Some time after the drug was used, Tatiana began breathing on her own for the first time in her life.
"If it wasn't for that drug then there would be nothing left to do for her," Elise Saiaan, Tatiana's mom, said to KIRO. "The gift of having her home is the only gift we wanted this Christmas."
Tatiana's parents hope their experience can help the drug gain approval by the FDA. The drug is already approved in Canada and Eurpoe.